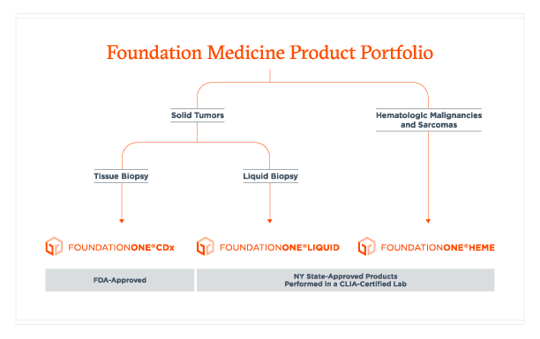
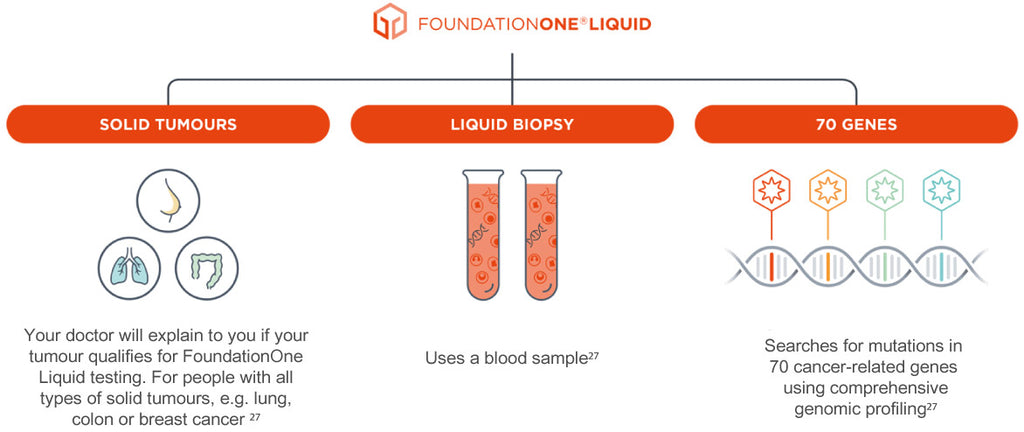
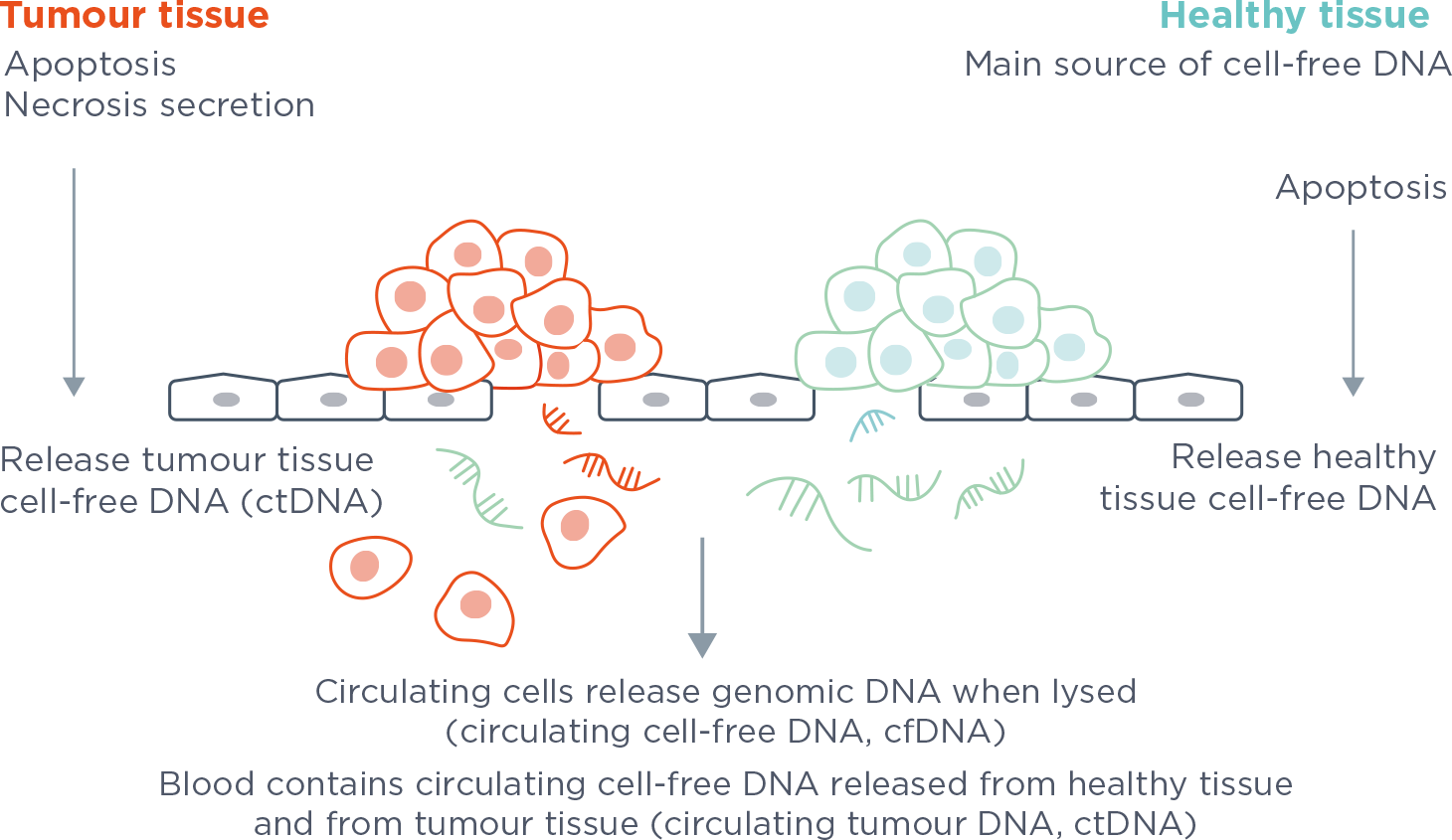
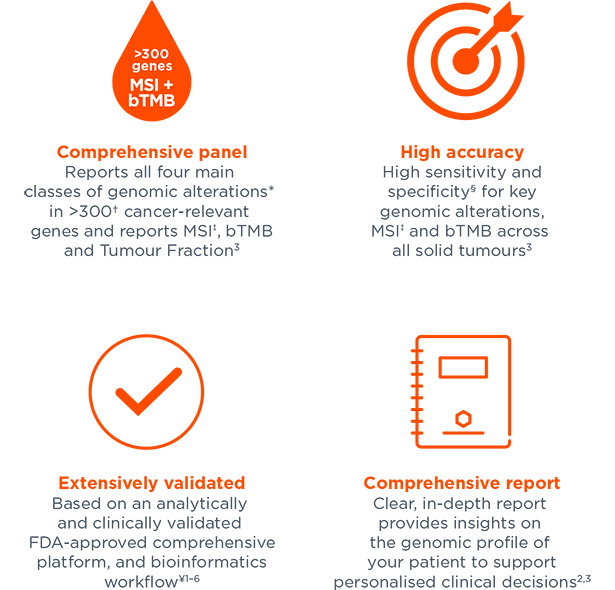
FoundationOne®Liquid CDx Companion Diagnostic Approved in NSCLC, Advanced Ovarian and Breast Cancer - Onco'Zine

Liquid Biopsy to Identify Actionable Genomic Alterations | American Society of Clinical Oncology Educational Book
FoundationOne®CDx Technical Information Foundation Medicine, Inc. 150 Second Street, Cambridge, MA 02141 Phone: 617.418.2200 I

Blood-based liquid biopsies for prostate cancer: clinical opportunities and challenges | British Journal of Cancer
Clinical and analytical validation of FoundationOne Liquid CDx, a novel 324-Gene cfDNA-based comprehensive genomic profiling assay for cancers of solid tumor origin | PLOS ONE