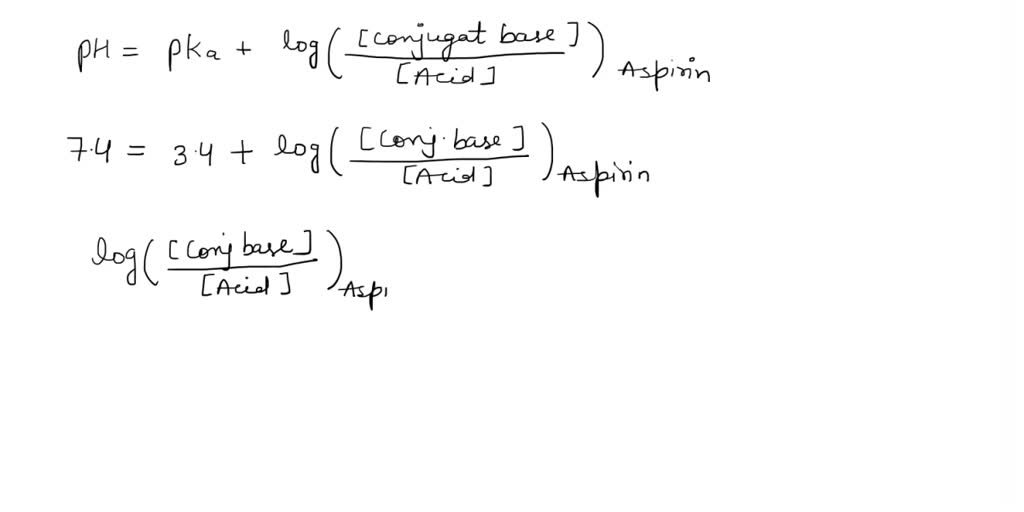
SOLVED: What is the ratio of aspirin's conjugate base to acidic form when in the blood (pH-7.4)? Aspirin has pKa of 3.4. A- : 10000 HA 1A- 3493 HA 10000 A : 1 HA 3493 A

SOLVED: The pKa for deprotonation of Aspirin is 3.5. Which form is predominant in the stomach (pH of stomach is 1)? a. The concentration of ionized form is predominant in the stomach.

Aspirin dissolution at pH 1.2 and 6.5 from polymer composite without... | Download Scientific Diagram

pH calculations and more in fundamentals of pharmaceutics. : Calculate pH of 250 ml solution containing 325 mg aspirin (acetylsalicylic acid).

Effect of aspirin on age at randomisation in PH and TD analysis for the... | Download Scientific Diagram
![Aspirin is a pain reliever with \\[{\\text{p}}{{\\text{K}}_{\\text{a}}}\\] = 2 . Two tablets each containing $0.09{\\text{ g}}$ of aspirin-are dissolved in 100mL solution. pH will be:\n \n \n \n \n A)0.5B)1.0C)0.8D)2.0 Aspirin is a pain reliever with \\[{\\text{p}}{{\\text{K}}_{\\text{a}}}\\] = 2 . Two tablets each containing $0.09{\\text{ g}}$ of aspirin-are dissolved in 100mL solution. pH will be:\n \n \n \n \n A)0.5B)1.0C)0.8D)2.0](https://www.vedantu.com/question-sets/5badb491-1213-4ab0-ad34-edc5746b30685272671716732010109.png)
Aspirin is a pain reliever with \\[{\\text{p}}{{\\text{K}}_{\\text{a}}}\\] = 2 . Two tablets each containing $0.09{\\text{ g}}$ of aspirin-are dissolved in 100mL solution. pH will be:\n \n \n \n \n A)0.5B)1.0C)0.8D)2.0
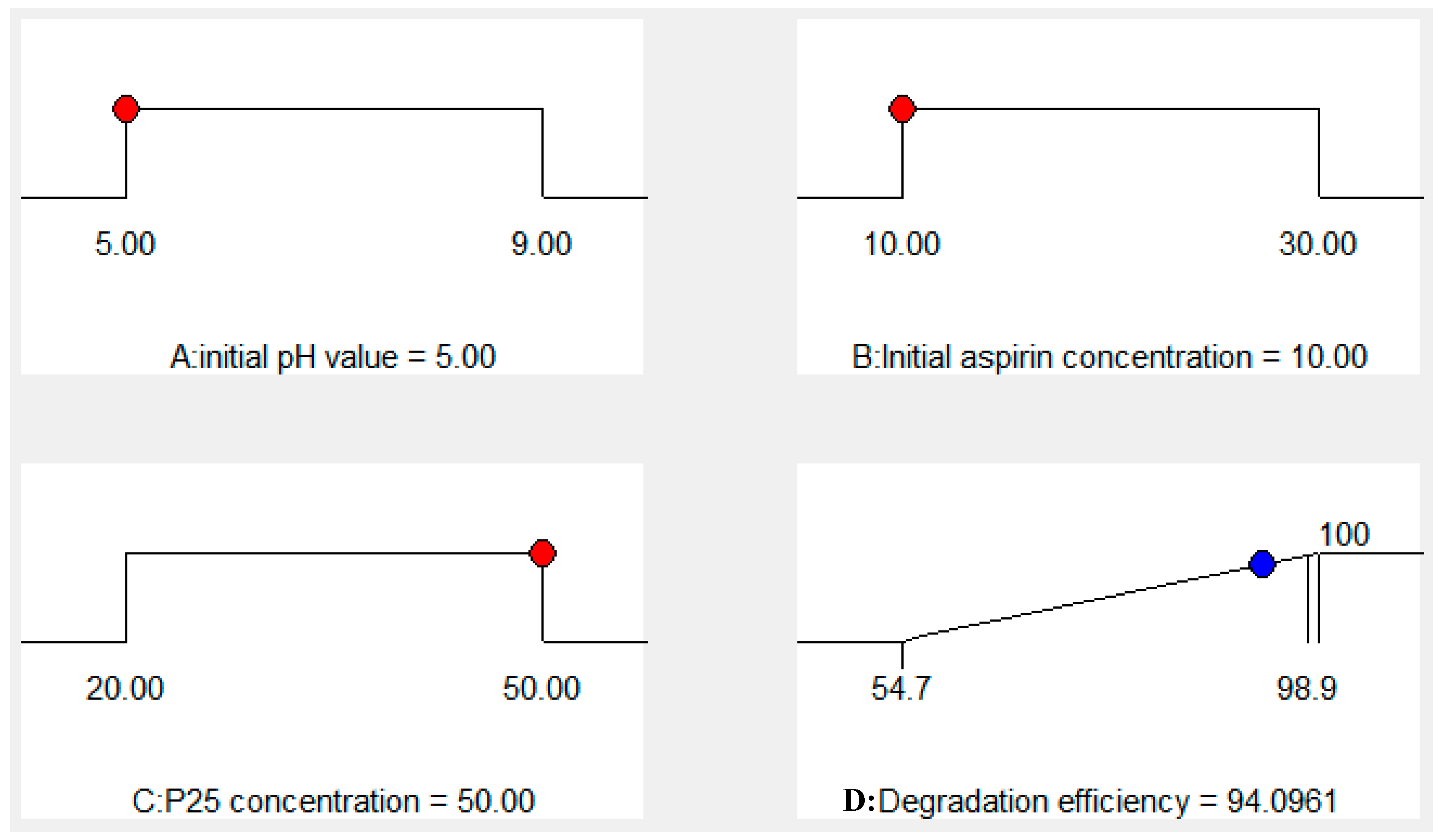
Catalysts | Free Full-Text | Photocatalytic Performance and Degradation Mechanism of Aspirin by TiO2 through Response Surface Methodology


![ASPILETS, Aspirin 80 mg 1 Chewable Tablet [PRESCRIPTION REQUIRED] | Watsons Philippines ASPILETS, Aspirin 80 mg 1 Chewable Tablet [PRESCRIPTION REQUIRED] | Watsons Philippines](https://api.watsons.com.ph/medias/zoom-front-10000066.jpg?context=bWFzdGVyfGltYWdlc3wyODc5NTh8aW1hZ2UvanBlZ3xhR1psTDJoalpDOHhNVEl5TkRVMU9UQTVNVGMwTWk5WFZFTlFTQzB4TURBd01EQTJOaTFtY205dWRDNXFjR2N8ZjdhYjFiYTZmNmMxNDIxNWZlNjI2ZTk4YWU2MTQzMDhjNzM0MDNmMjczZWFkNTM3NWViMTQxMjQzZjI5YjAyMw)